Contract
Manufacturing
Introduction
Leading Exporter of Unregistered and Registered Pharmaceutical Products With Contract Manufacturing Under WHO-GMP / EU-GMP / US-FDA / UKMHRA / PICS + more Approvals Manufacturing Unit
Our Company
The company provides quality products at affordable prices through our associate manufacturer from India. Initially company entered in the vital health care segments such as Analgesics/Anti-pyretics, Anti-inflammatory, Anti-parasitics, Anti-biotics and Vitamin drugs.
Export Introduction
In Premier we complete International Client’s requirement coming from UnRegulated markets or Regulated markets with Contract manufacturing and Third Party Manufacturing Supply having site registration with country specific MOH requirment or getting the New site cleared as as per the Specific Country MOH
Factory Approvals
HEALTH CANADA ,UK-MHRA ,EU-GMP, WHO-GMP, PICS, NAFDAC And More Added
International Recognised
ASIA – MOH-TAJIKISTAN, MOH-VIETNAM, MOH-MYANMAR, MOH-CAMBODIA, MOH-LAOS, MOH-MALDIVES, MOH-YEMEN, MOH-LEBANON AND MORE ADDED
AFRICA – MOH-MOZAMBIQUE, MOH-BURUNDI, MOH-MAURITIUS, MOH-NIGERIA, MOH-MAURITANIA, MOH-VENEZUELA
OTHER COVERAGE – MOH-POLAND, MOH-MEXICO, Caribbean – Islands And More added
Category
Anti- biotics / Anti-infective, NSAIDs, Gastro , Anti-viral, Anti-Fungal, Anti-Inflametory, Anti-malarial, CVS /CNS, Neuro / Psychiatric , Hepatic, Onclogy , Derma / skin care, Hormones / Steroids, Pre-filled Injections, Erectile Dysfuntion, EUffervesents tablets, Urology
Sections
Tablet , Capsules , Lotions , Injections , Dry Syrups, Drops , Sachets , Metered Dose Inhalers
In-House Dossier Team + COPP
Premier have in-house Dossier team with best of the class skilled knowlede in Dossier solution for export registration
Dossier as per European CTD / ASEAN CTD / non-CTD (country specific guideline) / E-CTD & COPP available product
Product List
View or Download The list by clicking below
Quality Objective
- To enhance customer satisfaction.
- To ensure proper dispatches of material at right time.
- To enhance the skill, knowledge of employees for better working.
- To ensure control over finished products.
- To launch 5 new products for export in every year.
Quality Policy
- Maximum emphasis is laid on quality assurance, which is of prime importance for PMA.
- We get manufacture our products with WHO-GMP-certified / EU-GMP-certified facilities only.
- Testing and manufacturing activities performed by competent, technical and experienced pharmacists.
- PMA has established Standard operating procedure (SOP) to implement maximum quality impact on day-to-day operations by closely monitoring procedures and implementing new ideas for better customer satisfaction and always improving quality management system
- Quality excellence is the foundation for our business management and the keystone of our goal, for customer satisfaction. It is therefore, our policy too
Vision
- To become a global pharmaceutical company within limited time frame, by providing a range of International quality products at competitive prices through integration.
- We will achieve our vision by closely working with valued Doctors, Partners, Company representative who are representing us in various countries.
- PMA wants to become front runner as recognized member of pharmaceutical society by providing best services.
DRUG LICENCE 21B

DRUG LICENCE 20B
INTERNATIONAL-IEC

PHARMEXCIL
.

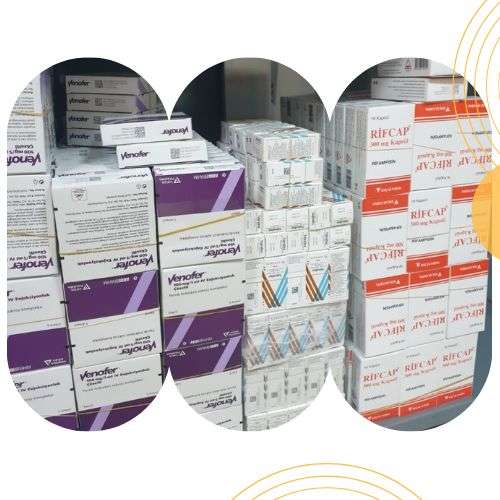
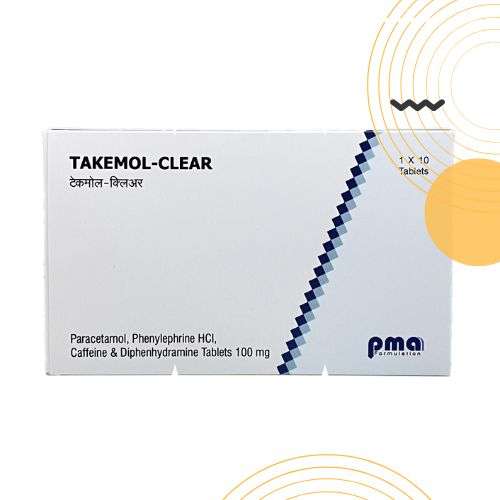
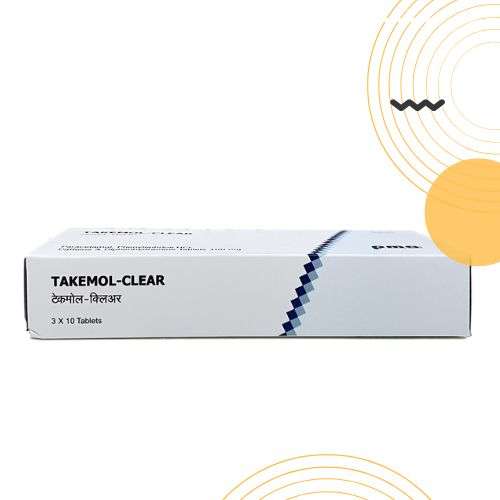
Product Photos
SECONDARY PACKAGING OF THE PRODUCTS WITH “QR” FACILITY




INTERNATIONAL CARGO PARCEL LABELLING AND TRANSPORT
PACKAGING OF THE PRODUCTS WITH “QR” FACILITY



STOCKING AND WAREHOUSE STORAGE FACILITY
PACKAGING AND RACKING STORAGE FACITLTIY OF THE PRODUCTS